THE POWER OF SOUND WAVES
The manufacturing of medical technology products such as implants, instruments and devices is subject to very strict requirements – including for parts cleaning. Ultrasound is an indispensable process here. It ensures that the required cleanliness is achieved in a stable, efficient and sustainable manner during both intermediate and final cleaning.
The combination of ultrasound and medical technology is primarily associated with diagnostic imaging. However, ultrasound can do much more in this area. Sound with frequencies above the human hearing range has established itself as an economical and sustainable standard process for wet-chemical cleaning applications in the manufacture and reprocessing of medical technology products such as instruments, implants and other devices. And it can be used for components made of a wide variety of materials such as stainless steel, titanium, cobalt-chrome alloys, ceramics and plastic.
Optimally Adaptable to the Task
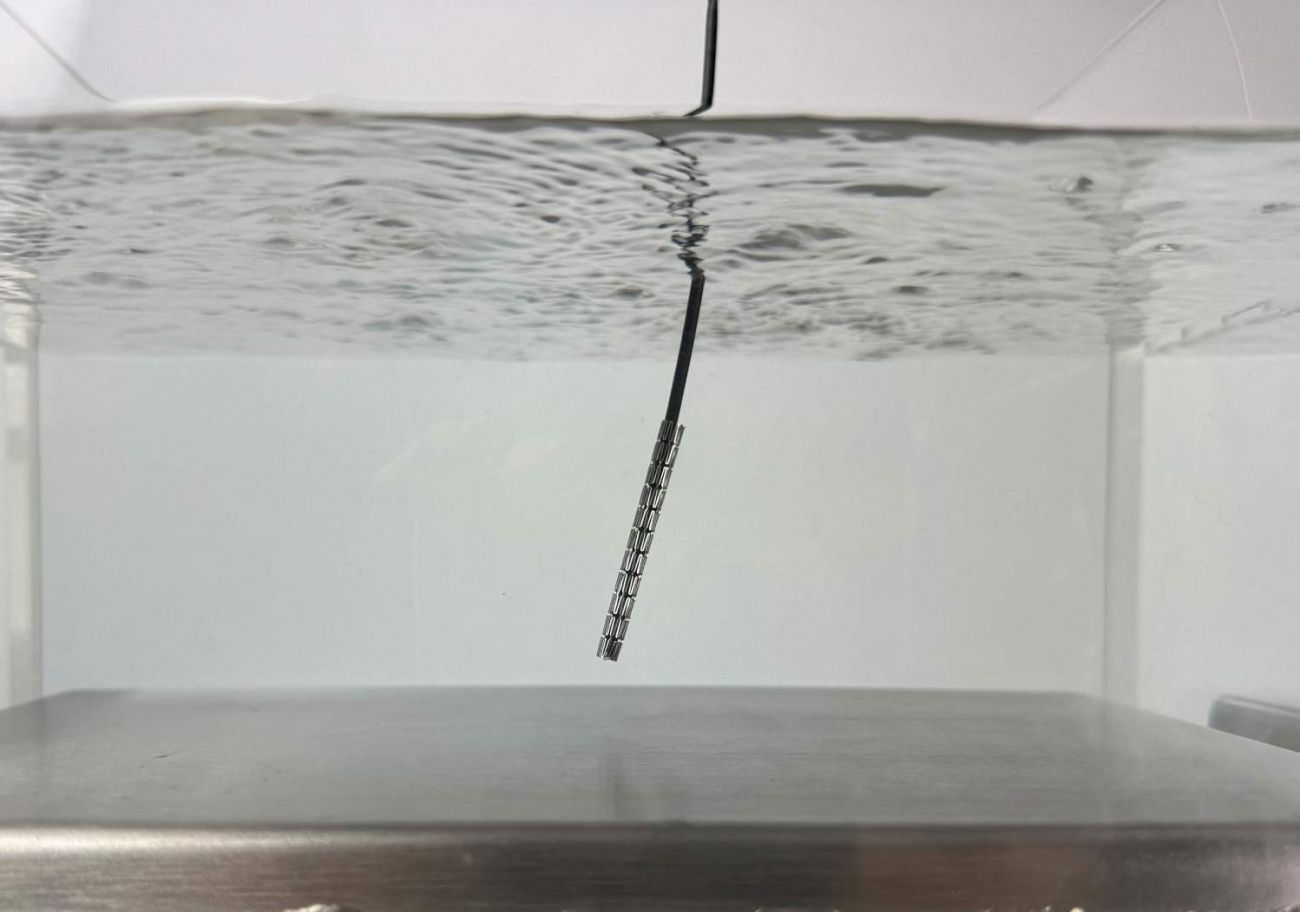 |
| The key criteria for stable and damage-free cleaning of the stents include a homogeneous sound field in addition to an optimally tuned ultrasound frequency and power. |
In cooperation with cleaning system manufacturers and users, Weber Ultrasonics develops custom-designed generators and transducer systems as rod, plate and immersible transducers with different frequencies for varied cleaning tasks in medical technology. This includes single-frequency solutions as well as dual- and multi-frequency ultrasonic systems in a frequency range from 25 to 132 kHz. They enable the construction of space-saving cleaning systems that are individually tailored to the workpieces and cleaning requirements. The development of vacuum-proof single, dual and multi-frequency immersible transducers also opens up the use of ultrasonic cleaning in full-vacuum cleaning systems. For high-purity applications, where particularly high demands are placed on component cleanliness and cleaning equipment, immersible transducers and plate transducers are available in a suitable design. With a surface roughness of < 0.35 µm, they meet the requirements of hygiene class 4 in accordance with DIN 11866.
Reliable Cleaning Stents
 |
| Stringent cleanliness requirements are placed on delicate implants such as stents. Gentle cleaning is ensured by ultrasonic cleaning. |
Heinz Schade, Managing Director, Weber Ultrasonics, also relies on the wide range of highly effective ultrasonic components. Founded in 1999 and based in Reutlingen, the company develops and produces machines for balloon and catheter production as well as stent processing, which are sold worldwide. In addition, there is software that is optimally adapted to various machines and, among other things, enables seamless documentation and traceability of processes required in medical technology. This helps to ensure that the strict requirements of the MDR with regard to process and product safety and quality management are met.
One focus of the system portfolio is on manufacturing steps for stent production that follow laser cutting, such as electropolishing, heat treatment, pickling, and ultrasonic cleaning. The company has been manufacturing tanks for cleaning systems in-house for around 20 years. This allows them to be adapted to various products and the specific requirements of each customer.
|
|
| For products with an open-pored sponge structure, with combined porous and polished surfaces, and with very fine capillaries, ultrasound can be combined with pressure cycling. |
"With our own production facilities, we were looking for a supplier for the ultrasonic components. In Weber Ultrasonics, we found a partner that has impressed us to this day with its expert advice and seamless cooperation," reports Schade.
To ensure the quality and safety of the products, stents are usually cleaned between various processes. Final cleaning is then carried out before packaging, typically in a cleanroom. "Increasingly stringent surface cleanliness requirements must be met. At the same time, it is essential to ensure that the sensitive stents are not damaged during cleaning," says the Managing Director, specifying the requirements. The key parameters for this process are the frequency of the ultrasound and the power in watts per liter of bath volume. Additionally, a homogeneous sound field must be generated in the bath to ensure consistently stable results. "The ultrasonic solutions from Weber Ultrasonics are ideal for these tasks. The power can be set very precisely by controlling the generator and the sound output is constant and reliable. We have had no problems with the ultrasonic systems so far," adds Schade.
 |
| Ultrasonic cleaning is the standard procedure for medical technology products such as instruments, implants, or other devices with very high cleanliness requirements. |
|
In cooperation with cleaning system manufacturers and users, Weber Ultrasonics develops custom-designed generators and transducer systems as rod, plate and immersible transducers with different frequencies for varied cleaning tasks in medical technology. This includes single-frequency solutions as well as dual- and multi-frequency ultrasonic systems in a frequency range from 25 to 132 kHz. |
Particulate and Film-Chemical Impurities are Removed
 |
| The tanks of the cleaning systems are adapted to the products to be cleaned and equipped with ultrasound. |
Ultrasound develops its cleaning effect in a liquid bath through the physical effect of cavitation: the electrical signals generated by an ultrasound generator are transmitted into the liquid through oscillating elements. The sound pressure is characterized by alternating negative and positive pressure phases. During the negative pressure phases, microscopically small cavities form and then collapse (implode) in the subsequent positive pressure phase. This process generates shock waves with considerable energy that ‘blast off’ particulate and film-chemical contaminants. At the same time, microcurrents are created in the liquid, flushing away detached or dissolved contaminants. These effects enable the removal of contaminants not only from the surface but also from complex geometries, cavities, holes, and structures.
For Critical Surfaces in Combination with Pressure Cycling Processes
For components such as additively manufactured implants with an open-pored sponge structure, combined porous and polished surfaces, or very fine capillaries, ultrasound can be combined with pressure cycling processes. In these vacuum flood cleaning processes, alternating negative and positive pressure, along with cavitation effects, are generated through regularly repeated pressure changes. This enables cleaning and rinsing media to reach areas that would otherwise be difficult or impossible to access.
| For components such as additively manufactured implants with an open-pored sponge structure, combined porous and polished surfaces, or very fine capillaries, ultrasound can be combined with pressure cycling processes. |
Source: Weber Ultrasonics AG





 Facebook
Facebook.png) Twitter
Twitter Linkedin
Linkedin Subscribe
Subscribe